Wednesday, October 31, 2012
This is immature...
 |
| Based on this blogpost |
Look -- there's a lot to be criticized about modern industrial agriculture and how it does things. But chemophobia and fear, uncertainty and doubt attacks are really annoying.
But what do I know? I'm one of the people who makes money from chemicals.
P.S. Bittman's "Minimalist" recipes are, indeed, pretty great. I like them a lot.
NPR covers #chemjobs in the RTP area
Listening to All Things Considered on the way home yesterday, a fascinating #chemjobs story in the middle of a series on financial success (and lack thereof). It's based out of North Carolina's Research Triangle area, so it's not a surprise that there are pharma/chemical industry ties:
That two of three people formerly connected with the chemical/pharma industries were laid off is no surprise, I suppose. While both people seem to have landed on their feet (and Dr. Zepp seems genuinely happy about his new job), one wonders if, in better economic times for our sector, whether they would have stayed in their positions.
Best wishes to all of us.
Donald Zepp, 67, and his wife, Carmen — 26 years his junior — have a 4-year-old son together. Both have been married before and both were well-employed before. Donald taught at Cornell and worked for the multinational agrichemical company Rhone-Poulenc until he was downsized. Carmen worked in the finance department of the pharmaceutical firm GlaxoSmithKline but quit after surviving ovarian cancer.
"It was eight years before I joined the legion of people who on getting out of corporate America say, 'That was the best that ever happened to me,' " Donald says. "It took eight years, but I did reach that point where one day I said, 'You know, I'm really happy, this beats the hell out of working.' "
The Zepps live in Wendell, N.C., 20 miles east of Raleigh, where they bought the local music store. Together, they've gone into business selling banjos. [snip] The Zepps cheerfully describe their economic situation as "dismal" and say they get by, for the most part, on his pension and Social Security. [snip]
...Phil Luby is another refugee from corporate life who struck out on his own. He says he used to make $200,000 a year marketing pharmaceuticals. When he was downsized in 2008, he rolled the dice on the used car business. It was a tough business to get into then, but he says things are improving.Dr. Zepp appears to have been laid off from Rhone-Poulenc from a position as a Ph.D. entomologist. I don't wish to speculate too much on Dr. Zepp's career, but one imagines that he tried at least a little bit to find other work in his field. The same goes for Mr. Luby, I'll bet.
That two of three people formerly connected with the chemical/pharma industries were laid off is no surprise, I suppose. While both people seem to have landed on their feet (and Dr. Zepp seems genuinely happy about his new job), one wonders if, in better economic times for our sector, whether they would have stayed in their positions.
Best wishes to all of us.
Process Wednesday: data comes from the weirdest places
There sure seem to be a lot of articles about working with hydrazoic acid safely in Organic Process Research and Development recently; all to the good! In the latest one (González-Bobes et al. [1]), the authors perform an "enantioselective palladium-catalyzed desymmetrization of a meso-bis-ester using TMS-N3. They do quite a bit of work towards making sure that there's not too much hydrazoic acid in the headspace -- after all, it's both terribly toxic and explosive. From the article:
[1] González-Bobes, F.; Kopp, N.; Li, L.; Deerberg, J.; Sharma, P.; Leung, S.; Davies, M.; Bush, J.; Hamm, J.; Hrytsak. M. "Scale-up of Azide Chemistry: A Case Study." Org. Process. Res. Dev. ASAP. DOI: 10.1021/op3002646
To maintain safety during downstream processing, a high pH and/or near complete reaction conversion must be achieved. Since the process required an acidification for intermediate stabilization (approximate pH 3.5), our control strategy relies on achieveing high reaction conversions (low residual free azide.) Therefore, the next step in the quantitative analysis becames the determination of the accetable residual free azide level after the palladium-catalyzed desymmetrization reaction. To accomplish this, we first interpreted data from the PUREX process, a nuclear fuel reprocessing method which forms HN3 as a byproduct. Using this data, we established a gas phase limit of 0.625 vol % which is based on the enriched condensate LEL in equilibrium wit the solution and gas phases.As a relative youngster, I'm not really aware of the PUREX process, so I thought I would take a look at it. It's pretty remarkable stuff, being able to extract out uranium and plutonium from spent nuclear fuel with tributylphosphate. Here's an excerpt from a random ORNL paper from 1977 on using hydrazine (the source of the hydrazoic acid) in the PUREX process:
In order to separate uranium and plutonium from each other in PUREX processing of nuclear reactor fuelds, advantage is taken of the fact that uranium (VI) is readily extracted from nitric acid solutions into the tributylphosphate-diluent organic phase while plutonium (III) is relatively inextractable. The uranium and plutonium are usually coextracted from the nitric acid fuel dissolver solution as uranium (VI) and plutonium (IV) and are then differentially stripped. The plutonium is reduced to plutonium (III) which strips into a dilute nitric acid solution leaving the uranium (VI) in the organic phase to be subsequently stripped with water. In some flowsheets, the plutonium is reduced prior to the uranium extraction in order to achieve the desired separation.
Plutonium (III) is not stable in nitric acid and, without the addition of a holding reductant, rapid and complete reoxidation to plutonium (IV) may occur. This can lead to reextraction of the plutonium during reductive stripping and plutonium recycled within the countercurrent contacting apparatus... Holding reductants are routinely added to solutions of plutonium (III) to destroy the nitrous acid which is continously formed by radiolytic decomposition of nitric acid. Very few reagents have proven useful as holding reductants...
Hydrazine rapidly reactions with nitrous acid, and the favorable kinetics of this reaction make hydrazine a practical holding reductant. With excess hydrazine present, the condition prevailing when hydrazine is used as a holding reductant, the fast reaction
N2H4 + HNO2 -> HN3 + 2H2O
There's something amusing about a situation in which rocket fuel gets added to nuclear waste to generate a "relatively stable" explosive waste product. My (figurative) hat's off to Kelmers and Browning for an education in nuclear fuel reprocessing, and to González-Bobes et al. for leading me there.occurs which stoichiometrically yields hydrazoic acid, HN3. Hydrazoic acid is relatively stable in nitric acid solutions.
[1] González-Bobes, F.; Kopp, N.; Li, L.; Deerberg, J.; Sharma, P.; Leung, S.; Davies, M.; Bush, J.; Hamm, J.; Hrytsak. M. "Scale-up of Azide Chemistry: A Case Study." Org. Process. Res. Dev. ASAP. DOI: 10.1021/op3002646
Tuesday, October 30, 2012
Daily Pump Trap: 10/30/12 edition
Between September 25 and September 29, 91 new positions were posted on C&EN Jobs. Of these, 21 (23%) are academically connected and 62 (68%) are from Kelly Scientific Resources.
Sigh: More Kelly, more academic positions. When the academic positions go away, it's going to be all Kelly, all the time, if things don't pick up.
Cincinnati, OH: Shepherd Chemical Company is looking for a Ph.D. synthetic inorganic chemist for a product development position. I suspect it's entry-level to mid-career, but I can't quite tell.
Hmmm: TSG is a company that performs regulatory consulting for chemical companies; they're looking for a Ph.D. chemist with 5+ years experience to be a "senior managing chemist." Looks TSCA-related. (I wonder if the "Safer Chemicals Act" will never get off the ground to replace TSCA; unlikely, I'll bet.)
Berkeley, CA: The Pesticide Research Institute is searching for a Ph.D. chemist to be an associate scientist. Looks to be risk assessments on pesticides -- you know, the exact thing that gets drinks bought for you in the Bay Area. The pay looks a little paltry (50-70k), but maybe that was just to fill out the form.
Tips, angry comments, suggestions wanted
I don't often say this, but it's true. My e-mail address (chemjobber -at- gmaildotcom) is at the top left hand part of this page, and if you want to talk to me directly, feel free to e-mail.
I'm thinking of putting "coaching carousel" feature in the Ivory Filter Flask, to note the holes that are created by professors moving between schools. So, if you have gossip to share, I'm all ears. Confidentiality, of course, is guaranteed.
I'm thinking of putting "coaching carousel" feature in the Ivory Filter Flask, to note the holes that are created by professors moving between schools. So, if you have gossip to share, I'm all ears. Confidentiality, of course, is guaranteed.
Ivory Filter Flask: 10/30/12 edition
Between September 23 and September 29, there were 33 academic positions posted on the ACS website. The numbers:
Total number of ads: 33
- Postdocs: 2
- Tenure-track faculty: 29
- Temporary faculty: 1
- Lecturer positions: 0
- Staff positions: 1
- US/non-US: 29/4
Plattsburgh, NY: SUNY College at Plattsburgh is hiring an assistant professor of organic and general chemistry. 50k minimum -- woowoo! (Holy cow, never mind -- the median household income in the town is 28k. You should be just fine.)
Huntingdon, PA: Juniata College is looking for an assistant professor of physical chemistry.
Nanjing, China: It's rather interesting that Nanjing University is touting its new institute through ACS Careers, and its connection with a Nobel Laureate:
Total number of ads: 33
- Postdocs: 2
- Tenure-track faculty: 29
- Temporary faculty: 1
- Lecturer positions: 0
- Staff positions: 1
- US/non-US: 29/4
Davenport, IA: The St. Ambrose University is looking for an assistant professor, looking mainly at the analytical side.
Plattsburgh, NY: SUNY College at Plattsburgh is hiring an assistant professor of organic and general chemistry. 50k minimum -- woowoo! (Holy cow, never mind -- the median household income in the town is 28k. You should be just fine.)
Huntingdon, PA: Juniata College is looking for an assistant professor of physical chemistry.
Nanjing, China: It's rather interesting that Nanjing University is touting its new institute through ACS Careers, and its connection with a Nobel Laureate:
The Institute of Chemistry and BioMedical Sciences (ICBMS) which is led by Drs. Aaron Ciechanover (the 2004 Nobel Laureate in Chemistry) and Guigen Li, seeks applications for 4-6 tenure track and tenured faculty positions in chemistry and biology at all levels.When will the first name-brand US professor of chemistry get handed an institute in China? It's coming, I'll bet.
Monday, October 29, 2012
Best wishes to East Coast readers
...to all my East Coast readers, riding out Hurricane Sandy. Hope all is well, and that you and yours are safe.
My one clever comment of the day: "Somewhere on the East Coast in a chemistry building, there's a foreign postdoc in the lab, wondering "Where is everybody?"
"Academic publishes anti-cancer tool compound, details at 11"
Just got my new copy of The Atlantic Weekly, with the "Brave Thinkers" section. In it, there's an interesting and slightly hagiographic short profile of Professor Jay Bradner, a physician and chemical biologist (emphasis mine):
Two years ago, after Jay Bradner made a remarkable breakthrough—the discovery of a molecule that, in mice, appeared to trick certain cancer cells into becoming normal cells—he did something unusual. Instead of huddling with lawyers to file for a patent on the molecule, Bradner simply gave his work away. Hoping to get the discovery into the hands of any scientist who could advance it, he published the structure of the compound (called JQ1) and mailed samples to labs around the world. The move, he says, felt like “the more efficient way to do science—and maybe the more honorable way.”
The open-source approach Bradner adopted is revolutionary in a culture where discoveries are kept secret, often until they can be tested, manufactured, and sold as treatments—a cruelly long process in the face of the cancers he studies.
The monopoly on developing the molecule that Bradner walked away from would likely have been worth a fortune (last year, the median value for U.S.-based biotech companies was $370 million). Now four companies are building on his discovery—which delights Bradner, who this year released four new molecules. “For years, drug discovery has been a dark art performed behind closed doors with the shades pulled,” he says. “I would be greatly satisfied if the example of this research contributed to a change in the culture of drug discovery.”Walked away! A fortune!
I was even more amused to read a somewhat different description of the compound from C&EN's Carmen Drahl in 2010: "We consider JQ1 a tool compound,” Knapp says. “It allows us to study how these readers participate in the development of disease.” Dana-Farber has filed for patents on (+)-JQ1 derivatives that might inspire drugs to treat diseases." Recent readers of C&EN will remember that JQ1 has other bioactivity as well: "In a paper published last month, the scientists showed that JQ1 causes reversible infertility in male mice (Cell, DOI: 10.1016/j.cell.2012.06.045). Now Bradner and Matzuk plan to use JQ1 as a lead compound to produce a second generation of compounds that are specific to BRDT."
I believe (and hope!) that most of the tone of this profile comes from Dan Morrell, the author of the piece, who may not be familiar with the unlikely odds and various hurdles (oral bioavailability issues, as noted in Prof. Bradner's TED talk on JQ1) that any one particular compound faces on its way to becoming a drug.
Prof. Bradner should be commended for his science and his willingness to rapidly publish his work*, but he need not be praised for walking away from a pile o' pharma cash. It may not have been there in the first place.
*As well as providing samples of JQ1! For one reason or another, this long-time aspect of biology (the mailing of plasmids and the like) has not penetrated broadly into chemistry. (Of course, it probably has something to do with our culture of I-can-make-it-myself.)
C&EN on flame retardants
This week in C&EN, a great set of articles on (drumroll) flame retardants. It makes for some pretty interesting reading, especially William Schulz's profile of Dr. Arlene Blum, one of the most prominent activists (and chemists -- she did her doctorate with Bruce Ames) against brominated flame retardants:
However, I'm less than convinced by the evidence of toxicity (I would say that, wouldn't I?). But that hasn't stopped activists from making bold claims about neurotoxicity of flame retardants or making suggestions that sound like terrible tradeoffs (from Cheryl Hogue's article on EPA's approach to BFRs):
Readers, been igniting your couches recently? What are your thoughts on flame retardants?
Fire-safety experts and other people who oppose Blum’s campaign—and who have called for a more reasoned analysis of the fire-safety science on flame retardants—say she brushes them aside as disqualified to provide expert opinion because of current, previous, or inconsequential chemical industry ties. They say Blum and her supporters shout them down at public workshops or refuse to engage in dialogue over the issues and the science. Worse, they say, Blum is promoting false information about fire safety from flame retardants via a high-visibility media campaign that stokes fear of chemicals that have been used for decades and have saved the lives of tens of thousands of people in the U.S. who have been the victims of home fires...
[snip] Blum and other scientists insist that a growing body of evidence indicates that the brominated flame-retardant chemicals used in upholstered furniture may, in some cases, be endocrine disrupters or have neurological and other health effects that make them unacceptable for use in everyday objects like sofas and chairs. Such flame retardants, she says, tend to accumulate in tissues and have been detected in the blood of adults and children.
“There are some 3,700 peer-reviewed papers on flame-retardant chemicals’ toxicity,” Blum says emphatically. Children especially, she says, should have very limited—if any—exposure to compounds that might damage their physical and intellectual development or leave them more vulnerable to other chronic health problems...
[snip] When asked if there could be differing interpretations of what is a large and complex body of data on the efficacy of flame retardants, Blum snaps, “I am not a fire scientist. This is such a broad field. There are about 10 disciplines involved.”
Blum says that other ways to prevent deaths from upholstered-furniture fires—improved building codes, reduced cigarette smoking, and increased use of sprinkler systems, for example— undermine the case for using flame-retardant chemicals.Similar to Dr. Blum, I don't really fully understand all the fire science behind flame retardants. Certainly, it would seem that both cigarette smoking and fire incidents are being statistically reduced over time, and that maybe flame retardant use could follow. It also doesn't help the situation when the chemical industry has resorted to less-than-honest tactics when promoting flame retardants.
However, I'm less than convinced by the evidence of toxicity (I would say that, wouldn't I?). But that hasn't stopped activists from making bold claims about neurotoxicity of flame retardants or making suggestions that sound like terrible tradeoffs (from Cheryl Hogue's article on EPA's approach to BFRs):
“Alternatives abound,” says Kathleen A. Curtis, national coordinator for the Alliance for Toxic-Free Fire Safety. The group is a coalition of activists who support techniques to reduce or eliminate the need to add flame-retardant compounds to products. Alternatives include increased use of sprinklers and smoke detectors, fire-safe cigarettes, and hiring more firefighters, she says. Improvements in product design are also an option, such as use of inherently fire-resistant materials—nonwoven polyester fibers, for example—or barriers between highly flammable materials such as foams and the outer fabric coverings of furniture, Curtis tells C&EN.Urrrr? That sounds quite expensive, and unlikely to happen (what's the likelihood over the next 20 years that we (as a country) are going to be hiring more, as opposed to less firefighters?)
Readers, been igniting your couches recently? What are your thoughts on flame retardants?
Friday, October 26, 2012
Woodward's Last Route
Death of A Project
Does anyone have a good way to cope with the sudden loss of a big project? We all know that it can happen in this business: IP doesn't work out, if trials fail, or customers change suppliers. I am reminded (depressingly, of course) of Robert Scott's comments after he found that Amundsen has made it to the South Pole first:
Also, which Kubler-Ross stages should I be dealing with? (Kidding!)
It is a terrible disappointment, and I am very sorry for my loyal companions. Many thoughts come and much discussion have we had. To-morrow we must march on to the Pole and then hasten home with all the speed we can compass. All the day dreams must go; it will be a wearisome return.Readers, what can I learn from the loss of a big project? Before I start tossing my samples in the Big Red Can, anything that I should be doing?
#ChemCoach: Over 40 entries!
If you haven't already, I really encourage you to go look at See Arr Oh's #ChemCoach carnival It's been a really interesting mix of people, both students and people who have long since graduated. Here's Day 1, Day 2, Day 3 and Day 4. Don't forget a note from amazing chemist and chemical historian Jeff Seeman.
It has been interesting for me to note how many of these people are former, not current, Big Pharma-employed. Those personally affected by layoffs at some point in their career is non-zero (20%?); I'll bet the number that have seen layoffs in their organization is basically 100%. It's a sobering reality of the economic times that we live in.
[Of course, there's probably quite a bit of sampling bias going on here; it's heavy on folks who read blogs or who are on Twitter, which is probably not representative of the overall population of working chemists.]
Also, friend of the blog Janet Stemwedel has made a good suggestion. Like any of the #ChemCoach entries? For each one you like, consider giving a $1 to the DonorsChoose Chembloggers challenge to further chemical education in K-12.
Again, if you haven't already, head over there and give it a read.
It has been interesting for me to note how many of these people are former, not current, Big Pharma-employed. Those personally affected by layoffs at some point in their career is non-zero (20%?); I'll bet the number that have seen layoffs in their organization is basically 100%. It's a sobering reality of the economic times that we live in.
[Of course, there's probably quite a bit of sampling bias going on here; it's heavy on folks who read blogs or who are on Twitter, which is probably not representative of the overall population of working chemists.]
Also, friend of the blog Janet Stemwedel has made a good suggestion. Like any of the #ChemCoach entries? For each one you like, consider giving a $1 to the DonorsChoose Chembloggers challenge to further chemical education in K-12.
Again, if you haven't already, head over there and give it a read.
Thursday, October 25, 2012
Why "Change the Equation" is wrong-headed about its definition of STEM
Thanks to a posting from the Chemistry Grad Student and Postdoc Blog, I was introduced to the interesting organization "Change the Equation", which is dedicated to increasing U.S. standards in STEM education. I would have happily supported or ignored this older Huffington Post blogpost about the issue, except for these interesting comments from their CEO, Linda Rosen:
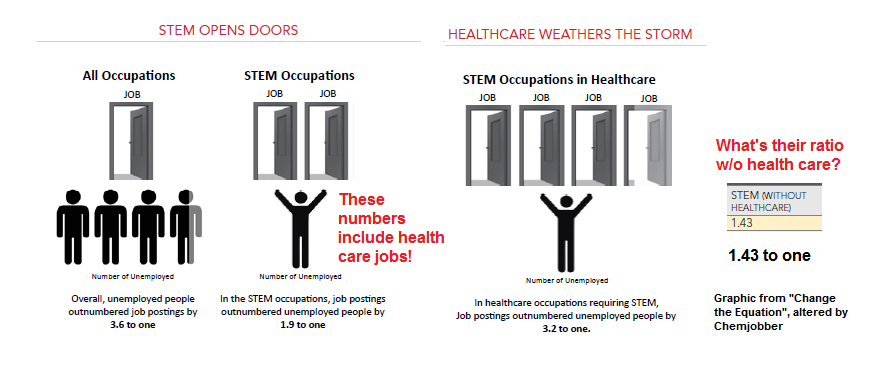
(Am I crazy, or is that a completely meaningless ratio? Am I wrong in thinking that, according to their ratio, an unemployed chemist is being measured against an job opening for cardiac surgery? It's also remarkable that they're associating job growth in some STEM fields (i.e. chemistry, or physics) with the completely ridonkulous increases in some health care fields that are going to be required to keep up with our aging population.)
I am sympathetic to people who are concerned about the quality of the American workforce and their level of STEM expertise. It seems self-evident to me that better STEM education is worthwhile. However, that doesn't justify questionable categorizing by Change the Equation, or the confusion that it will engender on the part of their audiences. While health care is important and contains some science and mathematics skills, I believe that it falls well outside the definition of "STEM."
*Look, you might need some math and some biology to be a licensed practical nurse (probably just solid arithmetic, really), but it doesn't make it a STEM job.
But for those with a science, technology, engineering and mathematics (STEM) background the picture is much brighter. Across the STEM fields, job postings outnumbered unemployed people by almost 2-to-1. Even in a tough economy, STEM is where the jobs are.
...The demand for STEM skills extends well beyond STEM-specific jobs, and the number of jobs requiring a STEM background is expected to have grown 17 percent between 2008 and 2018, far faster than the 10 percent growth projected for overall employment.Ms. Rosen is referencing the data in their report "STEM Help Wanted", where they reveal an interesting and unusual definition of "STEM" (emphasis mine):
There is no single, universally accepted definition of what constitutes a STEM-specific job. Our definition is broader than some, in that it encompasses those healthcare and management occupations that require strong STEM skills. We feel this broader definition allows us to offer a fuller account of the demand for STEM talent.
Our definition includes Computer and Mathematical occupations, Architecture and Engineering occupations, Life and Physical Science occupations, several Management occupations in STEM fields, and select Healthcare Practitioner and Technical occupations. In 2011, there were about 13.6 million people in these jobs, and they comprised about 11 percent of the total workforce.I find this to be a terribly problematic redefinition. Here is their methodology table; I broke it down in this Google spreadsheet. If you look at the numbers of current STEM occupation holders, CtQ has basically doubled the size of the available pool, from around 7 million positions to close to 14 million. It should also be pointed out that it appears to me that their definition of STEM jobs in healthcare appears to encompass 85% of health occupation job holders.* Also, according to their own numbers (see above), the introduction of the healthcare field dramatically changes the ratio of STEM openings to STEM unemployed.
(Am I crazy, or is that a completely meaningless ratio? Am I wrong in thinking that, according to their ratio, an unemployed chemist is being measured against an job opening for cardiac surgery? It's also remarkable that they're associating job growth in some STEM fields (i.e. chemistry, or physics) with the completely ridonkulous increases in some health care fields that are going to be required to keep up with our aging population.)
I am sympathetic to people who are concerned about the quality of the American workforce and their level of STEM expertise. It seems self-evident to me that better STEM education is worthwhile. However, that doesn't justify questionable categorizing by Change the Equation, or the confusion that it will engender on the part of their audiences. While health care is important and contains some science and mathematics skills, I believe that it falls well outside the definition of "STEM."
*Look, you might need some math and some biology to be a licensed practical nurse (probably just solid arithmetic, really), but it doesn't make it a STEM job.
Daily Pump Trap: 10/25/12 edition
Good morning! Between October 23 and October 24, there were 38 new positions posted on the C&EN Jobs database. Of these, 14 (37%) are academically connected and 16 (42%) are from Kelly Scientific Resources.
Memphis, TN: St. Jude Research Children's Hospital is searching for a B.S./M.S./Ph.D. research technologist; protein biochemistry expertise desired.
Columbus, OH: Ashland is looking for a principal scientist for polymer chemistry research; Ph.D. desired, but not required.
Naperville, IL: Cabot Microelectronics is looking for a senior research scientist; Ph.D. and 8 years of experience is desired. Also desired: "understanding the fundamental science of colloidal science, inorganic chemistry, surface science, particles, or polymers and their chemical reactions."
A broader look: Monster, Careerbuilder, Indeed and USAjobs.gov show (respectively) 249, 705, 2588 and 9 positions for the search term "chemist."
Memphis, TN: St. Jude Research Children's Hospital is searching for a B.S./M.S./Ph.D. research technologist; protein biochemistry expertise desired.
Columbus, OH: Ashland is looking for a principal scientist for polymer chemistry research; Ph.D. desired, but not required.
Naperville, IL: Cabot Microelectronics is looking for a senior research scientist; Ph.D. and 8 years of experience is desired. Also desired: "understanding the fundamental science of colloidal science, inorganic chemistry, surface science, particles, or polymers and their chemical reactions."
A broader look: Monster, Careerbuilder, Indeed and USAjobs.gov show (respectively) 249, 705, 2588 and 9 positions for the search term "chemist."
Wednesday, October 24, 2012
On earnings reports, DuPont, Dow to cut 4,000 jobs
After some optimistic comments, comes the reality of earnings season. From Chemistry World's Andrew Turley:
To those people who are going to lose their jobs, best wishes to them, and to all of us.
The chemical industry was hit by news of nearly 4000 planned job cuts, and multiple site closures, yesterday as two iconic firms, DuPont and Dow, announced dramatic restructuring plans alongside their financial results for the third quarter (Q3) of 2012. Sales from continuing operations fell 9% to $7.4 billion in Q3 as a result of weaker than expected demand in the markets for titanium dioxide and photovoltaic materials, said chief executive Ellen Kullman.
DuPont said it would reduce its global workforce by 1500 (2%) over the next 12–18 months with the aim of saving $450 million (£280 million) in annual costs. The move will cost the company $152 million in 2012.
[snip] Meanwhile, Dow said it would cut 2400 jobs, representing 5% of its global workforce, and close 20 manufacturing plants, in a bid to shave $500 million off its annual costs over the next two years. Sales at Dow fell 10% – 7% when adjusted for recently sold business activity – to $13.6 billion in Q3, with economic instability in Europe leading to lower prices cited as a key factor.The DuPont press release does not mention the actual cuts to come, while the Dow press release actually delivers the butcher's bill:
Dow will shut down a high density polyethylene facility in Tessenderlo, Belgium, a sodium borhidrate [CJ's note: sic] plant in Delfzijl, the Netherlands, as well as a number of Performance Materials manufacturing facilities, including: an Automotive Systems Diesel Particulate Filters manufacturing facility in Midland, Michigan; Formulated Systems manufacturing facilities in Ribaforada, Spain, Birch Vale, United Kingdom and Solon, Ohio; and an Epoxy resins facility in Kina Ura, Japan. Additionally, the Company will record an impairment charge related to the write-down of Dow Kokam LLC’s assets, reflecting weak global demand for lithium-ion batteries; and will consolidate certain assets in its Oxygenated Solvents business, as well as shut down a number of other small manufacturing facilities. These actions are expected to take place over the next two years.While I'd like to take a moment to kick Andrew Liveris in the shins a bit, I just don't have the heart to do that. Suffice it to say that it is broadly confusing to me what is going on in the global economy right now. Things in the US economy seem to be bumping along fine (for example, both consumer sentiment and the ACC's Chemical Activity Barometer are up), Europe is still more or less hurting and Asia seems to be slowing. I have my skepticisms about Paul Hodges and his economic predictions, but his insistence that we live in a world defined by uncertainty and unpredictability rings true to me.
To those people who are going to lose their jobs, best wishes to them, and to all of us.
Process Wednesday: the peril of scaling up catalyzed reactions
Kilomentor has a fascinating post on "catastrophic failure" in the plant and goes on this interesting tangent about the dangers of scaling up a catalyzed reaction:
The probability of catastrophic failure is increased for catalyzed reactions of which, for example, enantioselective reactions are a prominent contemporary class. The special additional risk is that the catalytic system may be more easily shut down by small, even trace, impurities that are difficult to measure much less control. Put another way, a catalyzed reaction is susceptible to poisoning and this can lead to catastrophic failure of conversion with no easily identifiable cause.
Catalyzed reactions are inherently less rugged than the uncatalyzed because the catalytic substance by definition is used in lower than stoichiometric quantity and so would be disproportionately affected by a particular quantity of a catalyst poison. Impurities in the inputs to a catalytic process can also accelerate reaction. When they are not added, as after a switch to a different source of an input, the performance may deteriorate or fail. Neal G. Anderson wrote in Practical Process Research & Development, First Edition, pg. 194: “The importance of trace beneficial impurities may become evident only by failure of the reaction when using different lots of starting materials, reagents, or solvents.” Thus the recommendation to perform laboratory experiments with the same materials to be used in the plant goes double for catalyzed reactions and this includes chemicals used to wash and prep the reactor.That last part is a healthy reminder to me -- do I think that the plant uses Dawn soap and acetone to clean out the reactors? No? Hmmmm....
(What are examples of reactions that can be accelerated through impurities? I suppose there's the classic serendipity of the Nozaki-Hiyama-Kishi...)
Tuesday, October 23, 2012
#ChemCoach: Process chemist
SeeArrOh is running a really excellent carnival over at his blog, Just Like Cooking. It's called #ChemCoach, and it's oriented towards showing people the different jobs that chemists have, and how they got there. (Does it really need a picture of Pete Carroll?) It's excellent, and I wish I had thought of it myself.
Here's my (very mundane) contribution -- I think you should go over there and see what others have to offer.
My current job: Process development chemist. I work at a small chemical manufacturing plant in the R&D department.
What I do in a standard "work day": Since we're such a small company, everyone does a little bit of everything. I try to think of myself as a lab chemist first -- I have a hood, I run reactions, I work them up, etc. It's the pretty standard stuff. We try to develop processes for large orders of whatever organic compound our customers desire.
What is unusual about my job is that it's connected to a small chemical manufacturing plant. Because of this, we get to do all the things that would be usually handled by other departments. Once it is decided (and sometimes it is VERY quickly decided) that a process is ready to take to the plant, we write up our procedure as a batch production record, we edit it, send it through the approval process and train our operators to run the process. We are on call nearly 24 hours a day to monitor the progress and quality of the reactions and provide chemistry-related troubleshooting. (Ever been woken up in the middle of the night to have someone ask you about filtration? I have.)
The old saw about "long periods of boredom punctuated by brief moments of terror" really seems to apply to what I do. There's nothing quite like slowly, slowly watching the peaks show up (or not show up!) on the HPLC, or to input a bunch of data into an Excel spreadsheet and cringe while hitting "Enter" to see if your purity/assay is high enough. It can be just as intense as watching a kid being born (okay, maybe not.) That lots of money and lots of time ride on the results of in-process checks or quality analyses is something I'll never quite get over.
What kind of schooling / training / experience helped me get there?: I have an undergraduate degree and a doctoral degree in chemistry. Boooooring. What probably helped me get my current position is that I served a 2 year sentence worked 2 years at an even smaller company doing kilo-scale synthesis, quite often lugging the 20L rotovap bulbs and moving drums of hazardous waste myself. Want to know what it's like to help ship a couple thousand gallons of flammable waste by hand (and hand truck?) I'm your guy. Want to know what it's like to load a 55 gallon reactor with a double diaphragm pump like you're a man on a firehose? I'm your fella.
How does chemistry inform my work? Well, I'm a chemist, so it permeates everything I do. For me, the question should almost be written as "what somewhat irrelevant things do I get asked, because I'm a chemist and I'm the closest thing to an expert?" In that vein, I get asked about equipment a lot. Very rarely, I have a good answer. Mostly, I shrug and I ask the engineers.
A unique, interesting, or funny anecdote about my career: First, all the really excellent anecdotes about my very checkered career cannot be told on the internet.
There's a real joy to using the simple tools of chemistry to make decisions. A while back, I had the opportunity to change a process to make it work a little faster. To monitor the reaction in a 1000+ gallon reactor, we would pull a sample to check its progress. To do so, I would... run a TLC.
The reaction check finally came up in the batch production record at about 1 am (of course.) I was able to bring my TLC plates, chamber, mobile phase and run the TLC out in the plant, basically next to huge reactors actually doing the chemistry. It was funny to be doing a TLC in the middle of the night, waiting for this piece of 1930s technology to tell me what to do next.
Here's my (very mundane) contribution -- I think you should go over there and see what others have to offer.
My current job: Process development chemist. I work at a small chemical manufacturing plant in the R&D department.
What I do in a standard "work day": Since we're such a small company, everyone does a little bit of everything. I try to think of myself as a lab chemist first -- I have a hood, I run reactions, I work them up, etc. It's the pretty standard stuff. We try to develop processes for large orders of whatever organic compound our customers desire.
What is unusual about my job is that it's connected to a small chemical manufacturing plant. Because of this, we get to do all the things that would be usually handled by other departments. Once it is decided (and sometimes it is VERY quickly decided) that a process is ready to take to the plant, we write up our procedure as a batch production record, we edit it, send it through the approval process and train our operators to run the process. We are on call nearly 24 hours a day to monitor the progress and quality of the reactions and provide chemistry-related troubleshooting. (Ever been woken up in the middle of the night to have someone ask you about filtration? I have.)
The old saw about "long periods of boredom punctuated by brief moments of terror" really seems to apply to what I do. There's nothing quite like slowly, slowly watching the peaks show up (or not show up!) on the HPLC, or to input a bunch of data into an Excel spreadsheet and cringe while hitting "Enter" to see if your purity/assay is high enough. It can be just as intense as watching a kid being born (okay, maybe not.) That lots of money and lots of time ride on the results of in-process checks or quality analyses is something I'll never quite get over.
What kind of schooling / training / experience helped me get there?: I have an undergraduate degree and a doctoral degree in chemistry. Boooooring. What probably helped me get my current position is that I s
How does chemistry inform my work? Well, I'm a chemist, so it permeates everything I do. For me, the question should almost be written as "what somewhat irrelevant things do I get asked, because I'm a chemist and I'm the closest thing to an expert?" In that vein, I get asked about equipment a lot. Very rarely, I have a good answer. Mostly, I shrug and I ask the engineers.
A unique, interesting, or funny anecdote about my career: First, all the really excellent anecdotes about my very checkered career cannot be told on the internet.
There's a real joy to using the simple tools of chemistry to make decisions. A while back, I had the opportunity to change a process to make it work a little faster. To monitor the reaction in a 1000+ gallon reactor, we would pull a sample to check its progress. To do so, I would... run a TLC.
The reaction check finally came up in the batch production record at about 1 am (of course.) I was able to bring my TLC plates, chamber, mobile phase and run the TLC out in the plant, basically next to huge reactors actually doing the chemistry. It was funny to be doing a TLC in the middle of the night, waiting for this piece of 1930s technology to tell me what to do next.
Daily Pump Trap: 10/23/12 edition
Good morning! Between October 18 and October 22, there were 120 new positions posted on the ACS Careers database C&EN Jobs. Of these, 25 (29%) are academically connected and 73 (61%) are from Kelly Scientific Resources.
An announcement: Bannered at the top of the jobs database: "Starting October 22, the ACS Careers Jobs Database sports a new name and look. C&EN Jobs will continue to offer an extensive network of jobs and employment opportunities for chemists and scientific professionals around the globe. ACS members can continue to find valuable career resources—including career fairs, consulting, and workshops—at www.acs.org/careers."
(Folks, it's not really a new look (yet, anyway.) Why this change? I dunno.)
An announcement of my own: Blogging a database that's mostly irrelevant Kelly positions and academic jobs is no picnic. It's taken a while to realize that I really enjoy bloggingACS Careers C&EN Jobs (!) when it's a lot of positions that I know that people will be interested in, as opposed to quality assurance positions (nothing wrong with QA!) and professorships in Doha. I hope things turn around soon. If fall is corporate recruiting season, it is my sense that they are not going to ACS Careers.
Bucks County, PA: Polysciences is once again looking for a B.S./M.S. synthetic chemist. Hey, why are these people routinely advertising for the same position?Are they growing? No, they're just probably losing people. (Nope -- that's a different company in Illinois with a similar name. I have no idea why they're rehiring for the same position, then.)
Edwards Air Force Base, CA: ERC is a contractor at the Air Force Research Laboratory outside of Edwards Air Force Base; they're looking for a synthetic chemist. You're probably going to be working on "energetic materials" of some sort -- sounds like a fun job.
Albany, NY: The New York State Department of Health is looking for an experienced M.S./Ph.D. analytical chemist to do GC/GC-MS. Pay is 82-100k.
Ugh: Of course, the membership of the American Chemical Society has an experienced medical technologist who would like to work in Olivette, Missouri. I'm so glad you asked!
An announcement: Bannered at the top of the jobs database: "Starting October 22, the ACS Careers Jobs Database sports a new name and look. C&EN Jobs will continue to offer an extensive network of jobs and employment opportunities for chemists and scientific professionals around the globe. ACS members can continue to find valuable career resources—including career fairs, consulting, and workshops—at www.acs.org/careers."
(Folks, it's not really a new look (yet, anyway.) Why this change? I dunno.)
An announcement of my own: Blogging a database that's mostly irrelevant Kelly positions and academic jobs is no picnic. It's taken a while to realize that I really enjoy blogging
Bucks County, PA: Polysciences is once again looking for a B.S./M.S. synthetic chemist. Hey, why are these people routinely advertising for the same position?
Edwards Air Force Base, CA: ERC is a contractor at the Air Force Research Laboratory outside of Edwards Air Force Base; they're looking for a synthetic chemist. You're probably going to be working on "energetic materials" of some sort -- sounds like a fun job.
Albany, NY: The New York State Department of Health is looking for an experienced M.S./Ph.D. analytical chemist to do GC/GC-MS. Pay is 82-100k.
Ugh: Of course, the membership of the American Chemical Society has an experienced medical technologist who would like to work in Olivette, Missouri. I'm so glad you asked!
Ivory Filter Flask: 10/23/12 edition
Good morning! Between October 15 and October 22, there were 34 academic positions posted on the ACS Careers database. The numbers:
Total number of ads: 34
- Postdocs: 2
- Tenure-track faculty: 28
- Temporary faculty: 1
- Lecturer positions: 2
- Staff positions: 1
Davis, CA: The University of California, Davis is looking for an assistant professor of chemical biology.
Indianapolis, IN: The University of Indianapolis desires an assistant professor of chemistry; Ph.D. in analytical chemistry desired.
Ann Arbor, MI: The University of Michigan is hiring for Dow Sustainability Fellows. Your postdoc will need to work towards sustainability, I assume -- the fields are broad. Pay is decent at 52k/yr for 2 years with medical and dental (amazing!)
Charleston, SC: The Citadel is hiring an assistant professor of chemistry. (Shouldn't some intrepid reporter find out what it is like to teach chemistry at one of the country's 9 or 10 uniformed undergraduate institutions? I hear you can't fall asleep in class at the Naval Academy.)
Total number of ads: 34
- Postdocs: 2
- Tenure-track faculty: 28
- Temporary faculty: 1
- Lecturer positions: 2
- Staff positions: 1
- US/non-US: 30/4
Davis, CA: The University of California, Davis is looking for an assistant professor of chemical biology.
Indianapolis, IN: The University of Indianapolis desires an assistant professor of chemistry; Ph.D. in analytical chemistry desired.
Ann Arbor, MI: The University of Michigan is hiring for Dow Sustainability Fellows. Your postdoc will need to work towards sustainability, I assume -- the fields are broad. Pay is decent at 52k/yr for 2 years with medical and dental (amazing!)
Charleston, SC: The Citadel is hiring an assistant professor of chemistry. (Shouldn't some intrepid reporter find out what it is like to teach chemistry at one of the country's 9 or 10 uniformed undergraduate institutions? I hear you can't fall asleep in class at the Naval Academy.)
Daily Pump Trap: 10/16/12 edition
Between October 11 and October 16, there were 81 new positions. Of these, 29 (36%) were academically connected and 38 (46%) were from Kelly Scientific Resources.
Danbury, CT: Sealed Air is hiring a B.S. chemist (2+ years experience) for a research position in their Packaging Products division. Sounds like fun.
Pittsburgh, PA: PIA Innovations is a new startup; they're hiring a Ph.D.-level chemist to synthesize amino acid monomers. Sounds interesting. 70k -- how far would that get you in Pittsburgh?
Danbury, CT: Sealed Air is hiring a B.S. chemist (2+ years experience) for a research position in their Packaging Products division. Sounds like fun.
Pittsburgh, PA: PIA Innovations is a new startup; they're hiring a Ph.D.-level chemist to synthesize amino acid monomers. Sounds interesting. 70k -- how far would that get you in Pittsburgh?
Ivory Filter Flask: 10/16/12 edition
Between October 9 and 15, there were 29 academically related positions posted on the ACS Careers website. The numbers:
Total number of ads: 35
- Postdocs: 3
- Tenure-track faculty: 29
- Temporary faculty: 0
- Lecturer positions: 0
- Staff positions: 3
Des Moines, IA: Grand View University is looking for an assistant professor of organic chemistry and biochemistry.
Aliso Viejo, CA: Soka University of America is a school that's aimed at teaching students to interact with Asian cultures, looks like. They're looking for an assistant professor of chemistry. (Looks like you need to be able to teach a whole other subject...)
Stony Brook, NY: Stony Brook University is looking for an assistant professor of chemical biology.
Houston, TX: Rice University is looking for a postdoctoral fellow: "There is a Postdoctoral Research Associate position available in the Verduzco laboratory in the Department of Chemical and Biomolecular Engineering at Rice University in Houston, TX. The position is for the development of water-soluble polymers with improved stability in high-salt and high temperature conditions for enhanced oil recovery. The initial appointment is for a period of 12 months, renewable for up to 3 years, contingent on outstanding performance. The appointment can begin as early as November 1, 2012."
Total number of ads: 35
- Postdocs: 3
- Tenure-track faculty: 29
- Temporary faculty: 0
- Lecturer positions: 0
- Staff positions: 3
Des Moines, IA: Grand View University is looking for an assistant professor of organic chemistry and biochemistry.
Aliso Viejo, CA: Soka University of America is a school that's aimed at teaching students to interact with Asian cultures, looks like. They're looking for an assistant professor of chemistry. (Looks like you need to be able to teach a whole other subject...)
Stony Brook, NY: Stony Brook University is looking for an assistant professor of chemical biology.
Houston, TX: Rice University is looking for a postdoctoral fellow: "There is a Postdoctoral Research Associate position available in the Verduzco laboratory in the Department of Chemical and Biomolecular Engineering at Rice University in Houston, TX. The position is for the development of water-soluble polymers with improved stability in high-salt and high temperature conditions for enhanced oil recovery. The initial appointment is for a period of 12 months, renewable for up to 3 years, contingent on outstanding performance. The appointment can begin as early as November 1, 2012."
Monday, October 22, 2012
DuPont employee: "We’re going to be around for another 200 [years]."
In this week's employment article, Laura Cassiday covers biotech/chemical companies that are highly rated for employee satisfaction. Here's some DuPont Crop Protection chemists sounding hopeful:
“I’m always learning, which keeps my work really stimulating,” says Andrew E. Taggi, a synthetic organic chemist who works on fungicide and herbicide discovery. During his eight years at Crop Protection, he has gained experience in many fields beyond synthetic chemistry, including toxicology, plant pathology, and agronomy. Taggi notes that, should his research interests ever change, the diverse skills he’s learned at Crop Protection would facilitate his transfer to other chemical and biological divisions of DuPont.Crop Protection has a burgeoning pipeline and many scientists reaching retirement age, so [32-year DuPont veteran] Lahm anticipates a large hiring push over the next five years. “We’ll need new Ph.D.-level scientists, but we also need experienced chemists with bachelor’s and master’s degrees,” he says. “Sometimes people bring in skills learned in industry that can’t be picked up quite as well in graduate school or postdoctoral programs.”
Taggi notes that many DuPont employees choose to remain at the firm for their entire careers. His father, also a synthetic chemist, retired from DuPont in 2010 after 38 years with the company. The large network of DuPont employees and retirees in the area helps foster a sense of community and stability. “DuPont has been here for more than 200 years, and you know we’re going to be around for another 200,” Taggi says.Nice to know that DuPont might be hiring experienced chemists -- if true, that's welcome news. And that DuPont employees think the company will be around for another 200 years? Boy, for all of our sakes, I sure hope he's right.
Oh, Carl! Djerassi pours some serious cold water on male contraception
A couple weeks ago, Michael Torrice published an interesting article on the current state of research towards male contraceptives. Pretty mundane, right? Well, good ol' Carl Djerassi decides to weigh in in the letters section of this week's C&EN -- and boy, is he ticked (emphasis mine):
...The biggest problem is the fact that the reproductive span of a young man is two to three times longer than that of a 20-year-old woman, who, for instance, will not ask whether continued use of her Pill would affect her fertility at age 55 or even later, whereas many a 20-year-old man would require a guaranteed answer before he would reach for his Pill.
Providing an epidemiologically valid assurance to this and other concerns, notably with respect to sexual potency, would be exceedingly expensive, time-consuming, and open to all kinds of litigious pressures because erectile dysfunction and prostate gland problems increase with advanced age and would be blamed by many men on their Pill rather than on the facts of life.
This economic reality has led the pharmaceutical industry to totally abandon any work on male contraception. And it is invariably glossed over through shameless grantsmanship or shameful naïveté by academic scientists who in their animal research at times make some exciting scientific observation that they then prognosticate as a practical and reversible new male contraceptive in humans, ignoring the fact that such work would take easily two or more decades (thus cannibalizing any patent protection) and cost well over $1 billion in today’s dollars for realization. Who, exactly, would foot that bill?
Carl DjerassiPerhaps I'm a sunny-eyed optimist, but that something is simply not economically practical at the moment is no reason for the government not to throw a couple of megabucks at it, right? It's difficult not to see Dr. Djerassi's letter as a teeny-tiny bit of legacy protection. That said, he's far wiser, experienced and knowledgeable about this subject than I will ever be... so there's that.
San Francisco
"Chemical Angel Network" funds startups
This is a pretty cool thing to hear about. By Marc Reisch, from the pages of this week's C&EN:
The Angel Resource Institute, a nonprofit information clearinghouse for early-stage investing, lists 329 angel investor groups in North America. But only one, the Chemical Angel Network, specializes in providing seed capital for chemistry-related start-up companies.
Founded just five months ago, CAN is a group of high-net-worth individuals with a background in chemistry. They have linked up through CAN to gather intelligence and share insights on potential investments, explains Mark Vreeke, one of the network’s founders...
White, an inventor with a Ph.D. in inorganic chemistry from Texas Christian University, says chemists are uniquely positioned to understand both the science of a chemistry-related start-up and the financial challenges it faces.
Unlike software and information technology firms that don’t require a lot of infrastructure, chemical start-ups need lab space, vented hoods, scientific instrumentation, and ways to handle hazardous chemicals, White points out. Chemical start-ups also need more time to do experiments and refine their products than do software-related businesses.
CAN hopes to offer early-stage chemistry-related firms a lot more than just money. “We’ll be able to offer help on the technical and business side,” Vreeke says, “and we’ll be able to provide connections to the rest of the chemical industry.”It will be interesting to track whether or not this will actually launch successful companies -- I certainly hope so.
Friday, October 19, 2012
The delicate balance of a weekly lab meeting
Dan Buckland, an engineer undergoing medical school training, has an interesting look at how physicians, scientists and engineers look at failure . Naturally, the "scientist" section looks familiar:
I note Mr. Buckland's last comments about "what stops someone from being too cruel" is much too ideal. Sometimes, personal dynamics of research groups often allows one group member to challenge results without receiving much doubt in return. I think it is incumbent on the research adviser (or the meeting chair) to make sure that everyone's data and conclusions get the same level of healthy skepticism.
The Scientist: Most scientific groups have some variation of the “Weekly Lab Meeting.” In these small group sessions, a group member will often present some in-progress project or recent data. Confusing results and unexpected data are presented, with the hope that the group can provide technical support or advice for future experiments. If the results are unexpected, it is common to question if the experiments were performed properly and if all the appropriate controls were conducted in order to establish the validity of the tests. Here, the questions do not assume the competence of the presenter without data to support that the techniques were properly executed. This is one form of “peer-review” and is the basis for the quality control that goes on in science. In fact, the Royal Society’s (The UK’s Academy of Sciences) motto is “Nullius in verba,” which is roughly “Don’t take anyone’s word for it”, including your closest colleagues. These meetings can become very heated, but usually what stops someone from being too cruel is that they know they have to stand in front of the same group at some point in the future and present their own data. However, if you ask most academic scientists, they can usually tell you a story of a lab meeting where someone went too far and a grad student or post-doc was found crying in a cubicle later.In graduate school, there are a lot of different reasons to have "the weekly lab meeting." Group meeting is a great time to deal with housekeeping issues, to give group announcements and yes, to report on one's progress in the laboratory. Done right, it's a fantastic seminar course with one's graduate advisor -- if you're cooped up in a small room 2 hours once a week, you can't help but learn something from them. Group meetings are also some of the best places to learn scientific skepticism about both your results and others'.
I note Mr. Buckland's last comments about "what stops someone from being too cruel" is much too ideal. Sometimes, personal dynamics of research groups often allows one group member to challenge results without receiving much doubt in return. I think it is incumbent on the research adviser (or the meeting chair) to make sure that everyone's data and conclusions get the same level of healthy skepticism.
Live in the RTP area? Want to see the old Burroughs-Wellcome site?
Looks like the now-GSK, then-Burroughs Wellcome building (what an odd shaped building!) is open for tours tomorrow only. (Looks like 10 bucks) Sounds interesting -- other people's old labs are always more interesting than your own.
I wonder what drugs were discovered in the building?
I wonder what drugs were discovered in the building?
Thursday, October 18, 2012
Daily Pump Trap: 10/18/12 edition
Good morning! Between October 16 and October 17, there were 42 new positions posted on the ACS Careers database. Of these, 14 (33%) were academically connected and 25 (60%) were from Kelly Scientific.
Glum: I like days when I can report on a lot of new positions posted -- today is not one of those days.
Orange, TX: Invista (part of Koch Industries) would like to hire a GC technician with 2+ years of laboratory experience.
Is Amgen hiring chemists? Yes: A trip to Amgen's careers website indicates that they're hiring (one or two?) experienced medicinal chemists at their South San Francisco and Cambridge sties. (posting date October 5.) This is the first time that I've done company-specific searching where they've actually been hiring. (Hey! Didn't they just lay off a bunch of medicinal chemists late last year? Huge sigh.)
A broader look: Monster, Careerbuilder, Indeed and USAjobs.gov show 252, 706, 2660 and 10 positions for the search term "chemist."
Glum: I like days when I can report on a lot of new positions posted -- today is not one of those days.
Orange, TX: Invista (part of Koch Industries) would like to hire a GC technician with 2+ years of laboratory experience.
Is Amgen hiring chemists? Yes: A trip to Amgen's careers website indicates that they're hiring (one or two?) experienced medicinal chemists at their South San Francisco and Cambridge sties. (posting date October 5.) This is the first time that I've done company-specific searching where they've actually been hiring. (Hey! Didn't they just lay off a bunch of medicinal chemists late last year? Huge sigh.)
A broader look: Monster, Careerbuilder, Indeed and USAjobs.gov show 252, 706, 2660 and 10 positions for the search term "chemist."
Wednesday, October 17, 2012
When are opportunity cost arguments most applicable?
Yesterday, a great many chemistry bloggers answered Mr. David Bernstein's question (posed on the Washington Post's education blog) "Why are you forcing my son to take chemistry?" I cannot really add anything to their righteous responses, so I'll send you over to Ash's, Derek's, SAO's and Janet's.
[Won't someone speak up for the joys of memorization? Very first, I assume that Mr. Bernstein is deeply mistaken about general chemistry being all memorization. Second, does anyone actually teach chemistry by memorization? Third, I've always felt that remembering something was the byproduct of actual understanding of the concept. People don't like memorizing things that are "useless" -- like the ordered list of American presidents, their parties, dates of office that I was asked to memorize as a student of AP US History (a special demand of our excellent teacher -- hello, Dr. P!) But it's only a memorized list if... you don't understand the ebb and flow of American politics. Do you memorize the alphabet, or do you understand it? Hard to say. Did I memorize Jeff Saturday's jersey number? (63, btw) Not really.]
I felt the biggest pang of potential regret when I read Mr. Bernstein's final argument:
But the opportunity costs of one year of high school chemistry seem relatively low. While Mr. Bernstein seems to have stipulated that it's not just about chemistry, I don't understand how it is chemistry that HAS to be the marginal class that has disallowed his son to not take a course in creative writing or HTML coding. At the same time, the relative opportunity costs of taking a Ph.D. in the sciences seem potentially very high. While we can debate the amount of time Mr. Bernstein's son might spend taking chemistry, we can all agree that a rigorous science graduate program will consume all of one's attention for at least 4 to 5 years. That you could be doing something else during that time seems apparent.
I also think there is also a distinct difference between the life of a high school student (15-18 years old) and a potential graduate student (somewhere between 22 and 28). When you're a high school student, it seems like there are very few options that you should look at and deny yourself. (Perhaps professional athlete or professional entertainer?) When you're in your late 20s, I think you should be thinking very seriously about how you will be making a living, and what your chances of success might be. Denying yourself options in high school seems a little unwise; by going to graduate school in the sciences and pursuing a doctoral degree (and beyond!), I feel that by pursuing one path (academic/industrial science), you are continually choosing not to walk other paths or raise the cost of pursuing them later.
[Won't someone speak up for the joys of memorization? Very first, I assume that Mr. Bernstein is deeply mistaken about general chemistry being all memorization. Second, does anyone actually teach chemistry by memorization? Third, I've always felt that remembering something was the byproduct of actual understanding of the concept. People don't like memorizing things that are "useless" -- like the ordered list of American presidents, their parties, dates of office that I was asked to memorize as a student of AP US History (a special demand of our excellent teacher -- hello, Dr. P!) But it's only a memorized list if... you don't understand the ebb and flow of American politics. Do you memorize the alphabet, or do you understand it? Hard to say. Did I memorize Jeff Saturday's jersey number? (63, btw) Not really.]
I felt the biggest pang of potential regret when I read Mr. Bernstein's final argument:
There’s a concept in economics called “opportunity costs,” which you may not have learned about because you were taking chemistry instead of economics. Opportunity costs are the sacrifices we make when we choose one alternative over another. A family store may be turning a good profit by selling tomatoes, but it would turn a bigger profit if it used the same shelf space to sell cucumbers. There are opportunity costs of selling tomatoes.
When you force my son to take chemistry (and several other subjects, this is not only about chemistry), you are not allowing him that same time to take a public speaking course, which he could be really good at, or music, or political science, or creative writing, or HTML coding for websites.
Maybe he will learn something in chemistry somewhere along the way. But he will lose out on so many other more important opportunities, and so will our society, which will have deprived itself of his full contribution.As someone who routinely uses "opportunity cost" arguments against the "STEM graduate school is good for you" meme, I am concerned that perhaps Mr. Bernstein's argument sounds a little too familiar.
But the opportunity costs of one year of high school chemistry seem relatively low. While Mr. Bernstein seems to have stipulated that it's not just about chemistry, I don't understand how it is chemistry that HAS to be the marginal class that has disallowed his son to not take a course in creative writing or HTML coding. At the same time, the relative opportunity costs of taking a Ph.D. in the sciences seem potentially very high. While we can debate the amount of time Mr. Bernstein's son might spend taking chemistry, we can all agree that a rigorous science graduate program will consume all of one's attention for at least 4 to 5 years. That you could be doing something else during that time seems apparent.
I also think there is also a distinct difference between the life of a high school student (15-18 years old) and a potential graduate student (somewhere between 22 and 28). When you're a high school student, it seems like there are very few options that you should look at and deny yourself. (Perhaps professional athlete or professional entertainer?) When you're in your late 20s, I think you should be thinking very seriously about how you will be making a living, and what your chances of success might be. Denying yourself options in high school seems a little unwise; by going to graduate school in the sciences and pursuing a doctoral degree (and beyond!), I feel that by pursuing one path (academic/industrial science), you are continually choosing not to walk other paths or raise the cost of pursuing them later.
Process Wednesday: the headaches of low yields
From the august pages of Organic Process Research and Development, a team from Boehringer Ingelheim talks about yield in a review titled "The Eight Criteria Defining a Good Chemical Manufacturing Process" [1](doesn't leave much room for debate, does it?):
*Don't do that, it's not nice to be mean.
1. Dach, R.; Song, J.J.; Roschangar, F.; Samstag, W.; Senanayake, C.H. "The Eight Criteria Defining a Good Chemical Manufacturing Process." Org. Process Res. Dev. ASAP. dx.doi.org/10.1021/op300144g
Criterion 3: Yield. The ideal or theoretical yield of a chemical reaction would be 100%. According to Vogel, yields around 100% are called quantitative; yields about 90% are excellent; 80%, very good; 70%, good; 50%, fair; and yields below about 40% are considered poor. Purification steps such as distillation or recrystallization always lower the yield, and the reported yield usually refers to the yield of the final purified product...
[snip] The objective of the development chemist is to reach yields of 80% or above. Yields in the range of 70% and below are often considered unfavorable in the pharmaceutical business. The lower the yield, the larger are the number and relative amounts of side products, which can carry over to subsequent steps and potentially remain undetected. Since questions can be raised about the destiny of the unaccounted materials during regulatory inspections, particular considerations are given to the monitoring and fate of genotoxic impurities, and avoidance of genotoxic impurities has become an increasingly important synthetic design consideration. Finally, for low-yielding steps, the cleaning operations can become complex and more involved.To this novice process chemist, the reminder of the further problems with low yields (undetected side products, problems with cleanouts) are important. And now you have it -- when someone says "and this oxidation was carried out with an excellent yield of 83%...", you can remind them that, ahem, Arthur Vogel says that your yield was "very good."*
*Don't do that, it's not nice to be mean.
1. Dach, R.; Song, J.J.; Roschangar, F.; Samstag, W.; Senanayake, C.H. "The Eight Criteria Defining a Good Chemical Manufacturing Process." Org. Process Res. Dev. ASAP. dx.doi.org/10.1021/op300144g
Monday, October 15, 2012
Pumpkin flavoring -- is there any chemistry there?
 |
| The taste of fall (Credit: AftonApple.com) |
According to a seven-page PowerPoint from the MenuTrends database of the firm Datassential, “This year is on track to be one of the most active years for seasonal pumpkin menuing” and could top the 2011 record, when more than 60 pumpkin-related dishes appeared on the menus of America’s top 250 chain restaurants. (Datassential really gets into this stuff, further reporting, among other things, that in appetizers, pumpkin is more likely to be roasted than toasted or puréed.) Zero in on beverage menus, and the increase is even more striking: Pumpkin drink offerings have increased 400 percent during the past five years.
The weird thing about pumpkin’s rise to baconlike ubiquity is that pumpkin, on its own, is not a very appetizing food at all. A dense and stringy fruit, it needs the accompaniment of a lot of sugar and spices before it becomes particularly palatable. As a marketing tool, however, pumpkin is perfectly pitched for today’s eaters. The fact that it needs that extra flavoring? That’s a bonus, not a bug, as far as the restaurant business is concerned. A pumpkin dish, in the era of the locavore, has connotations of virtue—when you think of pumpkin, you think of something farm-grown and wholesome. That helps make it a permissible indulgence, even when what you’re eating is mainly just sugar and spice. Never mind the recipe realities—savor those associations! [snip]
...The secret of Starbucks’ Pumpkin Spice Latte, for instance, is that it’s just a latte spruced up with pumpkin-flavored syrup—connoisseurs cite cinnamon, nutmeg, clove. Dunkin’ Donuts takes the feint further, dropping the “spice” from the name, though that’s mostly what you’re tasting. But no matter: If a restaurant served actual pumpkin purée, the taste and texture might shatter customers’ illusions. “Pumpkin,” on the other hand, is delicious.It has long been my assumption that there was some sort of advance in flavor chemical manufacturing that allowed for this explosion in pumpkin flavoring. I was mentally picturing rough hard-hatted men in white coveralls, loading pallets of pumpkins into reactors, turning on the steam to the jacket, the oh-so-careful distillation of essence of pumpkin... Now all my dreams are shattered, now that I've learned that it's probably just the spice and the sugar that is so popular.
I know my readership in the flavorings industry is not large -- anyone care to comment whether there is a single molecule of pumpkin flesh in pumpkin flavoring?
Kevin Reed: a person with an interesting job
Some Sangji inside baseball...
In the sordid history of UCLA's PR response to the #SheriSangji case, UCLA vice chancellor of legal affairs Kevin Reed plays a central role. He was the first UCLA official to spread the "Sheri Sangji was an experienced chemist" myth and has acted as a spokesperson for UCLA with respect to the case.
I was amused to be reading a story on ESPN about UCLA basketball's NCAA troubles recently and found this little tidbit: (emphases mine):
In the sordid history of UCLA's PR response to the #SheriSangji case, UCLA vice chancellor of legal affairs Kevin Reed plays a central role. He was the first UCLA official to spread the "Sheri Sangji was an experienced chemist" myth and has acted as a spokesperson for UCLA with respect to the case.
I was amused to be reading a story on ESPN about UCLA basketball's NCAA troubles recently and found this little tidbit: (emphases mine):
The investigation has cast such a cloud over the program that UCLA vice chancellor of legal affairs Kevin Reed monitored UCLA's annual basketball media day Wednesday to make sure Howland and the players did not address any questions about the investigation.
So with less than a month to go before the season begins with the grand reopening of a refurbished Pauley Pavilion, the Bruins don't know if their two top prospects will be on the floor for the Nov. 9 season opener against Indiana State.
"I'm just not going to comment with anything having to do with the ongoing investigation," Howland said with Reed lurking close behind. "It's inappropriate and it's not fair to the players. It's just a matter of their confidentiality with respect to the process. We're moving forward and I'm very hopeful and very optimistic that everything is going to work out and just waiting for the process to unfold and take place."Every large institution has its guardians of their public image; it is interesting to see how they work.
Hydraulic fracturing wastewater and #chemjobs
Whatever you think of hydraulic fracturing, there's one thing for certain -- there are probably a lot of new jobs in the field. From this week's Chemical and Engineering News article by Melody Baumgardner (emphasis mine):
There’s a lot of water to treat. Hydraulic fracturing requires between 3 million and 5 million gal of water per gas well. The water is combined with fracturing chemicals and a sand or ceramic proppant and then pumped into the horizontal branches of the well. The proppant props open fractures in the shale, allowing gas that has been trapped for eons to flow out. After fracking, roughly 35% of the water returns to the surface as flowback in the first weeks. Additional liquid known as produced water—a mix of fracking fluid and groundwater—comes up with the gas for most of the life of the well.
Hydraulic fracturing got its start in western states, where oil and gas drillers pump untreated wastewater into nearby wells driven deep into porous rock. For decades, deep-well injection has been the first choice for disposal because of its low cost. But the Marcellus areas of Pennsylvania and West Virginia have a geology that is not suited to deep-well injection. To dispose of the water off-site would require around 40 truck trips every day for weeks or months. That is costly, and energy companies can literally wear out their welcome when using local roads.
In contrast, the goals of wastewater treatment are to reuse, recycle, or reduce the water that comes out of the well. Chemical firms that specialize in water treatment such as Kemira and Ecolab’s Nalco unit; equipment makers including GE and Siemens; and service providers, both large and small, customize their offerings depending on the water’s contents and where it is destined to go. The main consideration in selecting technologies, all agree, is cost.
With prices for natural gas at a historic low of less than $3.00 per thousand cu ft, energy firms are compelled to select the cheapest legal alternative. “My biggest competitor is a hole in the ground,” says Mark Wilson, marketing director for unconventional gas at GE Power & Water. “We are looking for more energy efficiency and lower capital costs.”I think one of the few bright spots in the #chemjobs field has been Nalco, which has been hiring consistently over the last few years. Read the whole thing, especially if you're interested in learning about some of the actual environmental consequences of hydraulic fracturing.
Arrrrrrggggghhh! Letters on Rudy Baum's tenure
I think it's rather humorous and fitting that this week's C&EN has devoted its letters section to readers reflecting on the end of Rudy Baum's tenure. I'd like to think that ol' Rudy enjoyed reading all of those letters.
Best (?) yet, the first letter is all "Rudy, thanks for publishing my letters all these years, but you've been wrong all along!"
Go over there and enjoy, if that's your thing.
P.S. The "Arrrrrgh!" is a reference to this post.
Best (?) yet, the first letter is all "Rudy, thanks for publishing my letters all these years, but you've been wrong all along!"
Go over there and enjoy, if that's your thing.
P.S. The "Arrrrrgh!" is a reference to this post.
Saturday, October 13, 2012
Lesson learned
For the love of all that is holy, do not take the month off of features that you do three times a week.
Ignore the two posts below, and go straight to the podcast. Or not - up to you.
Ignore the two posts below, and go straight to the podcast. Or not - up to you.
Ivory Filter Flask: 9/11/12 and 10/9/12 editions
Still more catch-up work (whew!) on the Ivory Filter Flask. Holy cow, there are a lot of academic positions posted.
9/11/12 numbers (between 8/28 and 9/10)
Total number of ads: 35
- Postdocs: 1
- Tenure-track faculty: 33
- Temporary faculty: 0
- Lecturer positions: 0
- Staff positions: 1
- Ratio of US/international positions: 33 / 2
10/9/12 numbers (9/25-10/8):
Total number of ads: 109
- Postdocs: 4
- Tenure-track faculty: 95
- Temporary faculty: 1
- Lecturer positions: 5
- Staff positions: 4
- Ratio of US/international positions: 105 / 4
9/11/12 numbers (between 8/28 and 9/10)
Total number of ads: 35
- Postdocs: 1
- Tenure-track faculty: 33
- Temporary faculty: 0
- Lecturer positions: 0
- Staff positions: 1
- Ratio of US/international positions: 33 / 2
10/9/12 numbers (9/25-10/8):
Total number of ads: 109
- Postdocs: 4
- Tenure-track faculty: 95
- Temporary faculty: 1
- Lecturer positions: 5
- Staff positions: 4
- Ratio of US/international positions: 105 / 4
Daily Pump Trap: 9/11/12, 9/18/12 and 10/2/12 editions
Doing a little catch-up work here, tallying up all the weeks that I missed recently. There is nothing quite like spending 2 hours or so going cross-eyed at academic and Kelly postings.
9/11/12 edition: Between 9/6 and 9/11, 49 jobs total, 23 (48%) academically connected, 26 (52%) from Kelly Scientific Resources.
9/18/12 edition: 9/13-9/17, 71 jobs total, 41 (58%) academically connected, 28 (39%) from Kelly Scientific Resources.
10/2/12 edition: 9/25-10/1, 118 jobs total, 63 (53%) academically connected, 33 (28%) from Kelly Scientific Resources.
September is a crazy, crazy month for academic positions, I tell you what.
9/11/12 edition: Between 9/6 and 9/11, 49 jobs total, 23 (48%) academically connected, 26 (52%) from Kelly Scientific Resources.
9/18/12 edition: 9/13-9/17, 71 jobs total, 41 (58%) academically connected, 28 (39%) from Kelly Scientific Resources.
10/2/12 edition: 9/25-10/1, 118 jobs total, 63 (53%) academically connected, 33 (28%) from Kelly Scientific Resources.
September is a crazy, crazy month for academic positions, I tell you what.
Friday, October 12, 2012
Podcast: "I, for one, Welcome our Biochemical Overlords", Matt Hartings and Chemjobber
So Matt Hartings of ScienceGeist and I got into a fairly heated Twitter debate about the 2012 Nobel Prize in Chemistry (awarded to Professors Lefkowitz and Kobilka for their work on GPCRs).
So I invited Matt to give me a call via Skype yesterday so we could discuss our differences, and we recorded the results -- enjoy:
By now, the traditional apologies for sound quality, etc. It's been lightly edited for ums, ahs and my incredibly halting speech. If you want a summary, Matt had better, more substantive arguments in general, and dealt with my counter-arguments easily. He will be using his persuasive powers to round up synthetic chemists to toil in protein crystallography laboratories.
0-4:00: Introduction
4:00: Did Lefkowitz and Kobilka do chemistry?
10:30: CJ's concerns about the trend of biochemical Nobelists
16:30: Whither the chemical enterprise?
24:15: The Dominance of Biochemistry
28:28: Will organic chemists ever win a Nobel Prize ever again?
30:30: Matt's "Ratatouille" analogy
32:00: CJ asks, "How much of bench-level molecular biology is chemistry?"
35:15: Matt gets CJ to cry uncle with the classic #debatefail of "I wasn't arguing that."
40:00: A long digression on the breadth of chemistry as a field
45:10: Matt does an excellent Carl Sagan impression, talking about the "supernova of chemistry."
47:30: Is it time for the Chemistry Diaspora? Matt: No.
49:00: Discussion of impact on chemical education
54:00: The disproportionate media impact of the Nobel
55:00: CJ notes that BLS-projected job growth for biochemists and biophysicists is quite high.
1:03:45: CJ feels a little better
1:05:00 Matt provides a nice summary.
So I invited Matt to give me a call via Skype yesterday so we could discuss our differences, and we recorded the results -- enjoy:
By now, the traditional apologies for sound quality, etc. It's been lightly edited for ums, ahs and my incredibly halting speech. If you want a summary, Matt had better, more substantive arguments in general, and dealt with my counter-arguments easily. He will be using his persuasive powers to round up synthetic chemists to toil in protein crystallography laboratories.
0-4:00: Introduction
4:00: Did Lefkowitz and Kobilka do chemistry?
10:30: CJ's concerns about the trend of biochemical Nobelists
16:30: Whither the chemical enterprise?
24:15: The Dominance of Biochemistry
28:28: Will organic chemists ever win a Nobel Prize ever again?
30:30: Matt's "Ratatouille" analogy
32:00: CJ asks, "How much of bench-level molecular biology is chemistry?"
35:15: Matt gets CJ to cry uncle with the classic #debatefail of "I wasn't arguing that."
40:00: A long digression on the breadth of chemistry as a field
45:10: Matt does an excellent Carl Sagan impression, talking about the "supernova of chemistry."
47:30: Is it time for the Chemistry Diaspora? Matt: No.
49:00: Discussion of impact on chemical education
54:00: The disproportionate media impact of the Nobel
55:00: CJ notes that BLS-projected job growth for biochemists and biophysicists is quite high.
1:03:45: CJ feels a little better
1:05:00 Matt provides a nice summary.
Taking your kids to work... on the weekends
One of the best parts of any of the scientific Nobel Prize is the human stories that arise. I really enjoyed Carmen Drahl's interview of novelist Cheryl Renée Herbsman, the daughter of 2012 chemistry Nobelist Professor Robert Lefkowitz:
CD: Growing up, what kinds of things did you hear from your father about what he worked on?
CRH: Growing up, I don’t think my siblings and I necessarily understood what our father was researching. We knew it had to do with receptors, but that might have been the full extent of our understanding. Sometimes he would talk at dinner about whether the research was going well or not. Occasionally he would take us to the lab with him on a Saturday morning, where we would have wheeled desk-chair races and explore the walk-in refrigerators. Often, we would hear him dictate papers into his Dictaphone. The words didn’t mean much to us. But I remember my younger sister writing up “scientific papers” of her own with a lot of important-sounding made-up words. My dad always ended the dictation by saying, “RJL etc.” So my sister ended hers with her initials, etc., as well.Some of the happiest memories I have of my childhood are going to my engineer father's workplace and wandering amongst the blueprints and computer terminals. (Also, the massive cups of super-concentrated hot chocolate that he would make me.)
I look forward to the day when I can take my kids to work with me on the weekend (safely, of course. They don't make steel-toed boots in kids' size, I don't think.)
Go on over there and read it.
AbbVie CEO and credential issues
I have been remiss in posting recently, so I missed posting about this news about the Abbott pharma spin-off, AbbVie and its new CEO's resume troubles last month. But yesterday's comments about fibbing on one's résumé reminded me. From Crain's Chicago Business last month:
Part of me thinks that this is not a huge deal. Mr. Gonzalez is from a time where it was not uncommon for people to join companies with simply a high school degree and work their way up to the top. (And there's an argument to be made -- what should we do with our educational system that this could be the case in the future? A debate for another time, and probably another blog.) Surely, his position as CEO is based on his performance as a manager and administrator.
But, of course, this can be seen as the very best fruits of credential fabulism. You fib a little to get into the door, and then you work your way to the top on your own merits!
Readers, can you split this baby?
Abbott Laboratories misstated the education credentials of Richard Gonzalez, the executive tapped to head its pharmaceutical spinoff, in at least nine regulatory filings between 2002 and 2007, Crain's has found.
Mr. Gonzalez did not receive a bachelor's degree in biochemistry from the University of Houston, nor a master's degree in biochemistry from the University of Miami, contrary to claims in Abbott's filings with the U.S. Securities and Exchange Commission when the longtime company executive was a director. He retired from Abbott as president and chief operating officer in 2007 but returned two years later.
In October, Mr. Gonzalez was named CEO of AbbVie, which is projected to have $18 billion in annual sales after it is spun off later this year. Without a college degree, he seemingly would be a rarity among CEOs of major corporations.What to say about this? Abbott claims that it was an error by administrative staff that allowed the error/mistake/lie (?) to become public record. It's beyond the scope of this blog to determine whether the error was intentional or not (I am sure someone has been desperately searching for copies of Mr. Gonzalez's resume from the early 1980s.)
Part of me thinks that this is not a huge deal. Mr. Gonzalez is from a time where it was not uncommon for people to join companies with simply a high school degree and work their way up to the top. (And there's an argument to be made -- what should we do with our educational system that this could be the case in the future? A debate for another time, and probably another blog.) Surely, his position as CEO is based on his performance as a manager and administrator.
But, of course, this can be seen as the very best fruits of credential fabulism. You fib a little to get into the door, and then you work your way to the top on your own merits!
Readers, can you split this baby?
Thursday, October 11, 2012
How much of people's resume/CVs are checked?
One of the recurring themes of fabulists is how often they fake their credentials to gain their positions. One of my favorite ones is the 2009 case where a Texas A&M administrator claimed to be a former Navy SEAL. From the Austin Statesman:
Readers, ever seen people claim to have things on their CVs that were not true?
(What's to stop someone from inserting their name into articles and padding their publications sections? "The Chemical Development of the Commercial Route to Sildenafil: A Case History." Dale, D.J.; Dunn, P.J.; Golightly, C.; Chemdued, F.A.K.; Hughes, M.L.; Levett, P.C.; Pearce, A.K.; Searle, P.M.; Ward, G.; Wood, A.S. Org. Proc. Res. Dev., 2000, 4 (1), pp 17–22.)
*There's a Sean Connery quote from "The Untouchables" that comes to mind.
Texas A&M's No. 3 administrator presented himself as a warrior-scholar: a former Navy SEAL with a doctorate from Tufts University. But records obtained by The Bryan-College Station Eagle indicate that Alexander Kemos never was part of the elite fighting force, and Texas A&M officials confirmed Friday that he doesn't have a doctorate or even a master's degree, which was a posted requirement for the $300,000-a-year position as the top adviser to Texas A&M President R. Bowen Loftin. Kemos resigned Friday morning, the day after he was confronted by Loftin in Maine, where they were vacationing.So it comes as no surprise to anyone that Annie Dookhan, the dry-labbing chemist, was caught giving herself a master's degree in chemistry. From Carmen's article in C&EN:
Her résumé lists a master’s degree in chemistry from the University of Massachusetts, Boston*, but she does not hold that credential according to school officials.Isn't it time that someone came up with a way of checking all the educational credentials that people report in their résumé and CV? If LinkedIn (for example) were to offer a "confirmation" checkmark the way that Twitter does with celebrities, I would think that jobseekers would be forced to verify their credentials (for a nominal fee, of course.) Are there résumé checking services? That'd would be a cheap way to avoid credential fabulists (or at least filter out the dumb ones...)
Readers, ever seen people claim to have things on their CVs that were not true?
(What's to stop someone from inserting their name into articles and padding their publications sections? "The Chemical Development of the Commercial Route to Sildenafil: A Case History." Dale, D.J.; Dunn, P.J.; Golightly, C.; Chemdued, F.A.K.; Hughes, M.L.; Levett, P.C.; Pearce, A.K.; Searle, P.M.; Ward, G.; Wood, A.S. Org. Proc. Res. Dev., 2000, 4 (1), pp 17–22.)
*There's a Sean Connery quote from "The Untouchables" that comes to mind.
Daily Pump Trap: 10/11/12 edition
Good morning! Between October 9 and October 10, there were 33 new positions posted on the ACS Careers website. Of these, 9 (27%) are academically connected and 13 (40%) are from Kelly Scientific Resources.
Camden, SC: Invista is looking for a B.S./M.S./Ph.D. laboratory manager for its polymer laboratory. Looks like a lot of analytical work.
Cleveland, OH: Promerus LLC is looking for a M.S./Ph.D. NMR chemist; they're a polymer/packaging company, looks like. 10+ years experience, industrial experience desired.
Chemistry Nobel qualified: SRI International is searching for a Ph.D. protein biochemist for a position in Harrisonburg, VA. Looks like PI-level qualifications desired. Strong mass spectrometry experience wanted.
Philadelphia, PA: CPS, Inc. needs a agriculturally-oriented formulator. B.S./M.S./Ph.D. desired, 5-10 years industrial experience. Rheology, agricultural biotechnology desired. Pay decent-ish: 70k-105k. (Okay, maybe not? Seems low.)
Camden, SC: Invista is looking for a B.S./M.S./Ph.D. laboratory manager for its polymer laboratory. Looks like a lot of analytical work.
Cleveland, OH: Promerus LLC is looking for a M.S./Ph.D. NMR chemist; they're a polymer/packaging company, looks like. 10+ years experience, industrial experience desired.
Chemistry Nobel qualified: SRI International is searching for a Ph.D. protein biochemist for a position in Harrisonburg, VA. Looks like PI-level qualifications desired. Strong mass spectrometry experience wanted.
Philadelphia, PA: CPS, Inc. needs a agriculturally-oriented formulator. B.S./M.S./Ph.D. desired, 5-10 years industrial experience. Rheology, agricultural biotechnology desired. Pay decent-ish: 70k-105k. (Okay, maybe not? Seems low.)
Wednesday, October 10, 2012
Why I am discomfited by today's chemistry Nobel
Congratulations to Professor Robert Lefkowitz and Professor Brian Kobilka for the 2012 Nobel Prize in Chemistry "for studies of G-protein-coupled receptors." Their biochemical and structural work is groundbreaking and fundamental in nature -- and immediately useful, too. As Derek said, "At least a third of marketed drugs, after all, are GPCR ligands, so their importance is hard to overstate."
There's been a bit of back-and-forth in the chemblogo/Twittersphere about whether or not this is another "biology" prize for the Chemistry Nobel, and whether or not that is a good thing or a bad thing. The most eloquent defenders of the position that it is indeed a good thing include the two most eloquent writers in the chemblogosphere, Derek Lowe and Ash of The Curious Wavefunction. From Derek:
There's been a bit of back-and-forth in the chemblogo/Twittersphere about whether or not this is another "biology" prize for the Chemistry Nobel, and whether or not that is a good thing or a bad thing. The most eloquent defenders of the position that it is indeed a good thing include the two most eloquent writers in the chemblogosphere, Derek Lowe and Ash of The Curious Wavefunction. From Derek:
Biology isn't invading chemistry - biology is turning into chemistry. Giving the prize this year to Lefkowitz and Kobilka takes us from the first cloning of a GPCR (biology, biology all the way) to a detailed understanding of their molecular structure (chemistry!) And that's the story of molecular biology for you, right there. As it lives up to its name, its practitioners have had to start thinking of their tools and targets as real, distinct molecules. They have shapes, they have functional groups, they have stereochemistry and localized charges and conformations. They're chemicals. That's what kept occurring to me at the recent chemical biology conference I attended: anyone who's serious about understanding this stuff has to understand it in terms of chemistry, not in terms of "this square interacts with this circle, which has an arrow to this box over here, which cycles to this oval over here with a name in the middle of it. . ." Those old schematics will only take you so far.
So, my fellow chemists, cheer the hell up already. Vast new territories are opening up to our expertise and our ways of looking at the world, and we're going to be needed to understand what to do next. Too many people are making me think of those who objected to the Louisiana Purchase or the annexation of California, who wondered what we could possibly ever want with those trackless wastelands to the West and how they could ever be part of the country. Looking at molecular biology and sighing "But it's not chemistry. . ." misses the point. I've had to come around to this view myself, but more and more I'm thinking it's the right one.In Ash's comments on the prize, he comments that he's tired with this ages-old debate:
I have to say that the whole “But is this chemistry?!” meme is getting quite boring. Binding of a small molecule to a GPCR is as much of a molecular interaction as anything in chemistry. Plus, think about the downstream chemistry that GPCRs do, including phosphorylation and salt-bridge breakage. I thought chemists were supposed to rub their hands with glee at the reduction of biology to chemistry while biologists fret and fume. But I see the opposite, biologists being quite sanguine about proteins being awarded medicine Nobels while chemistry continue to complain about proteins (chemicals!) being awarded chemistry Nobels. As I have said before though, this very bickering shows the astonishing reach and diversity of the field. If you can’t even agree on a definition for your field, well, that means your field is truly omnipresent.There are a number of questions/complaints that have been raised with this trend in the chemistry Nobels -- here are some that I have heard and agree with:
- While chemists may have a more-and-more expansive definition of chemistry, do biologists agree with this redefinition/annexation? What have biologists said about this?
- If we asked Professors Lefkowitz and Kobilka last week if they were chemists, would they have said yes or no? Is this relevant?
- This is chemistry's one day to bask in the media spotlight. We have emphasized biological chemistry for the 6 of the last 13 Nobels (h/t SeeArrOh). Is this the right mix?
- What does this trend tell graduate students in other, non-life-science-oriented chemistry fields?
- If you are using the tools of chemistry to do biology, are you a chemist? If you are not a chemist and yet advance the understanding of chemistry, should you be considered for the chemistry Nobel? (My answer: yes, certainly.)
- Isn't this just a sign that the Nobels, their strictures and the way they are awarded are a terrible way to highlight scientific merit and achievement? Should judged awards for science look more like the Heisman Trophy or the Westminster Dog Show?
My main complaint against this trend of biology/biologists winning recent chemistry Nobel prizes is that it is beginning to distract from the non-life-sciences aspects of chemistry. Physical chemists, inorganic chemists, analytical chemists, polymer chemists (and yes, organic chemists too!) are all losing this zero-sum game, and missing out on their opportunity to tell the larger public that they exist and why they're important to humanity. We can always rely on the public to be willing to support (and fund!) disease cures and advances in human biology (and they have their own prize for Physiology or Medicine!), but gaining public acceptance or recognition for these other (more fundamentally chemistry?) fields is much more difficult. I think that's why I wince a little bit when it's yet another life scientist that gets to step up to the mike.
Process Wednesday: solids blending and segregation
 |
| A child's delight, a manufacturer's problem. Credit: zoomdoggle.com |
In other applications the sample size subjected to scrutiny is not as clear cut. Take, for example, a box of cereal. In one specific example a manufacturer produced a cereal with a cereal particle and a marshmallow candy particle. The packaging line filled boxes from a series of conveyors that fed a mixture of two components into a gravimetric packaging machine that dispensed the required weight of the blend into each package. The two components of the mixture were of similar size and density and the only blending that occurred was in the handling system along several conveyors and in a surge hopper. Each component was meter onto the conveyor system, at the correct ratio, but there was not a discreet blending process. The manufacturing plants fine tuned their systems to get an acceptable mixture into each box.
In this case, each box was always within weight tolerance for the stated package quantity, but there was no direct control of the components in any box. In an effort to improve quality, a new packaging line was installed that handled each component individually up to the packaging head where each component was weighed in a series of weigh hoppers, and then each box was filled from several hoppers of each component, selected by the packaging system controlled, to control both the proportion of each component and the total weight in a box. This would seem an ideal solution since it would guarantee both accurate package weight and composition. From the producers point of view, they had improved quality by insuring that every package met their quality standard.
However, to everyone's surprise, consumer complaints from packages produced at this plant went up. What the plant had gained in compositional accuracy they had sacrificed in uniformity. Consumers found that individual bowls of cereal poured from the new packages had much wider variations of the two components than boxes filled from the old filling line. ...The new system allowed only about one second from the time the ingredients were separate until they were combined in the package. There was very little opportunity to do any blending, and the result was that some boxes were well-blended while others were segregated.This doesn't have a ton to do with "classic" process chemistry (how often are raw/fine chemical manufacturers blending solids?) -- I just thought it was kinda funny.
UPDATED: Added "raw/fine" and "classic" to clarify. Formulation and drug delivery, of course, does a lot of solids blending.
Subscribe to:
Posts (Atom)